Precision Spine AccuFit® Lateral Plate System. Catalog #58-VS-6560
- CataBlog

- May 22, 2022
- 1 min read
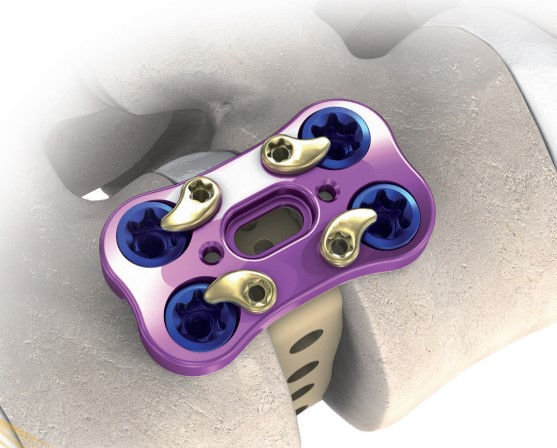
Vendor/Manufacturer: Precision Spine
Product/Product Line: AccuFit® Lateral Plate System
Vendor/Manufacturer Catalog #: 58-VS-6560
Global Unique Device (GUD) Primary Device Identifier Number: 00840019904636
Global Unique Device (GUD) Device Description:
Ø6.5mm Variable Screw, 60mm
Global Medical Device Nomenclature (GMDN) Preferred Term Name:
Spinal bone screw, non-bioabsorbable
Global Medical Device Nomenclature (GMDN) Definition:
A small, threaded rod with a slotted head intended to be used for internal spinal fixation by being screwed into the spine to hold a stabilization device (e.g., rod, plate) to bone; it is made of a material that is not chemically degraded or absorbed via natural body processes (includes implant grade metal such as surgical steel, titanium alloy, or carbon fibre). The device is available in various types, including pedicle and transfacet, and is typically used to provide immobilization and stabilization of spinal segments in the treatment of various spinal instabilities or deformities.
Category 1: Spine
Category 2: Thoracolumbar Plate & Screw Systems
Category 3: Screw
FDA Regulation Medical Specialty: Orthopedic Devices - Prosthetic Devices
FDA Regulation Number: 888.3060
FDA Regulation Description Classification Name:
Spinal intervertebral body fixation orthosis
FDA Classification Product Code: KWQ
FDA Classification Product Code Device Name:
Appliance, Fixation, Spinal Intervertebral Body
FDA Premarket Submission: K162211
The Precision Spine AccuFit® Lateral Plate System is
SUBSTANTIALLY EQUIVALENT to the following products:
For more information, please visit us at Infonomics.com




Comments