Precision Spine AccuFit® Lateral Plate System. Catalog #58-SC-1000
- CataBlog

- May 22, 2022
- 1 min read
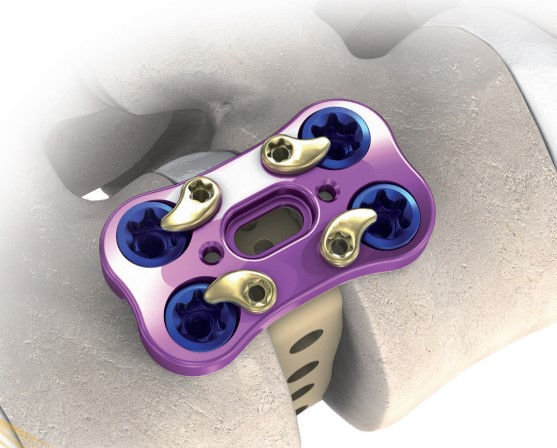
Vendor/Manufacturer: Precision Spine
Product/Product Line: AccuFit® Lateral Plate System
Vendor/Manufacturer Catalog #: 58-SC-1000
Global Unique Device (GUD) Primary Device Identifier Number: 00840019919876
Global Unique Device (GUD) Device Description:
AccuFit® LP Case
Global Medical Device Nomenclature (GMDN) Preferred Term Name:
Orthopaedic surgical procedure kit, non-medicated, reusable
Global Medical Device Nomenclature (GMDN) Definition:
A collection of various orthopaedic surgical instruments, dressings and the necessary materials intended to be used to perform an orthopaedic surgical procedure, however the kit is not dedicated to orthopaedic implantation. It does not contain pharmaceuticals. This is a reusable device.
FDA Regulation Medical Specialty: Orthopedic Devices - Prosthetic Devices
FDA Regulation Number: 888.3060
FDA Regulation Description Classification Name:
Spinal intervertebral body fixation orthosis
FDA Classification Product Code: KWQ
FDA Classification Product Code Device Name:
Appliance, Fixation, Spinal Intervertebral Body
FDA Premarket Submission: K162211
The Precision Spine AccuFit® Lateral Plate System is
SUBSTANTIALLY EQUIVALENT to the following products:
For more information, please visit us at Infonomics.com




Comments