NuVasive® MODULUS Interbody Fusion. Catalog #1225050P2
- CataBlog

- Dec 30, 2020
- 1 min read
Updated: May 11, 2024
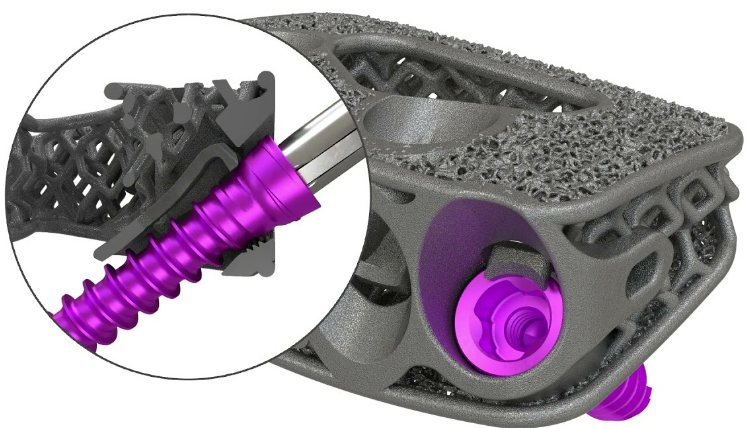
Vendor/Manufacturer: NuVasive®
Product/Product Line: MODULUS Interbody Fusion
Vendor/Manufacturer Catalog #: 1225050P2
Global Unique Device (GUD) Primary Device Identifier Number:
00887517726841
Global Unique Device (GUD) Device Description:
Modulus XLW, 10x22x50mm 15°
Category 1: Spine
Category 2: Interbody Fusion Devices
Category 3: Lumbar/T-Lumbar
Global Medical Device Nomenclature (GMDN) Preferred Term Name:
Metallic spinal interbody fusion cage
Global Medical Device Nomenclature (GMDN) Definition:
A device intended to be implanted into the space of an intervertebral disc that has been partially or totally removed during surgery in order to allow bone fusion between two contiguous vertebral bodies, typically in the treatment of degenerative disc disease (DDD). It is in the form of a hollow, porous, threaded and/or fenestrated cylindrical or geometrical device made of metal [e.g., titanium (Ti)] that provides mechanical stability and sufficient space for therapeutic spinal bone fusion to occur; bone graft is typically used to help with osseointegration. Fixation screws and devices associated with implantation may be included with the device.
FDA Regulation Medical Specialty:
Orthopedic Devices - Prosthetic Devices
FDA Regulation Number: 888.3080
FDA Regulation Description Classification Name:
Intervertebral body fusion device
FDA Classification Product Code(s): MAX, PHM
FDA Classification Product Code Device Name:
MAX: Intervertebral Fusion Device With Bone Graft, Lumbar
PHM: Intervertebral fusion device with bone graft, thoracic
FDA Premarket Submissions: K163230
The NuVasive® MODULUS Interbody Fusion Device is
SUBSTANTIALLY EQUIVALENT to the following product(s):
For more information, please visit us at Infonomics.com




Comments